|
|
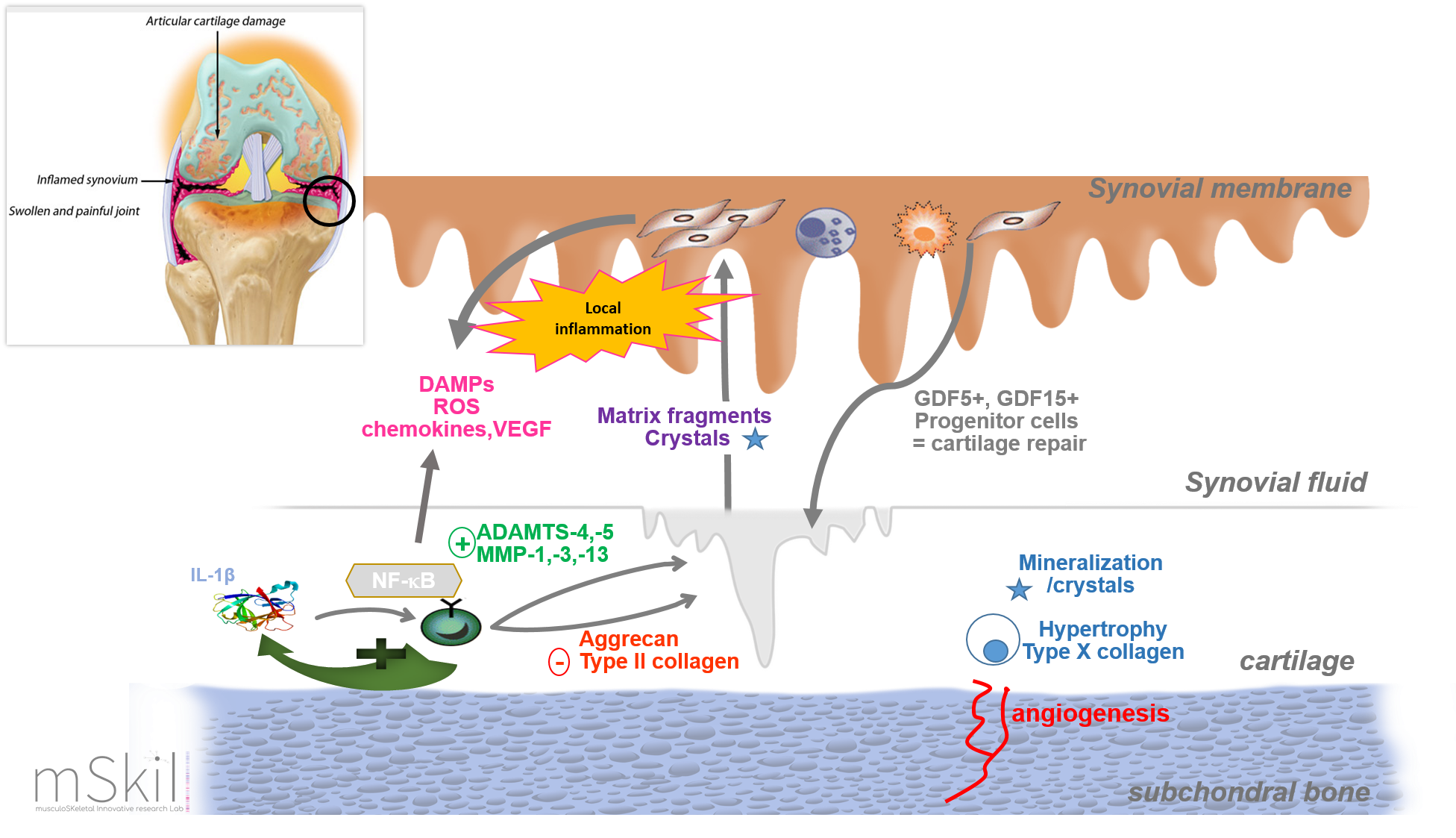
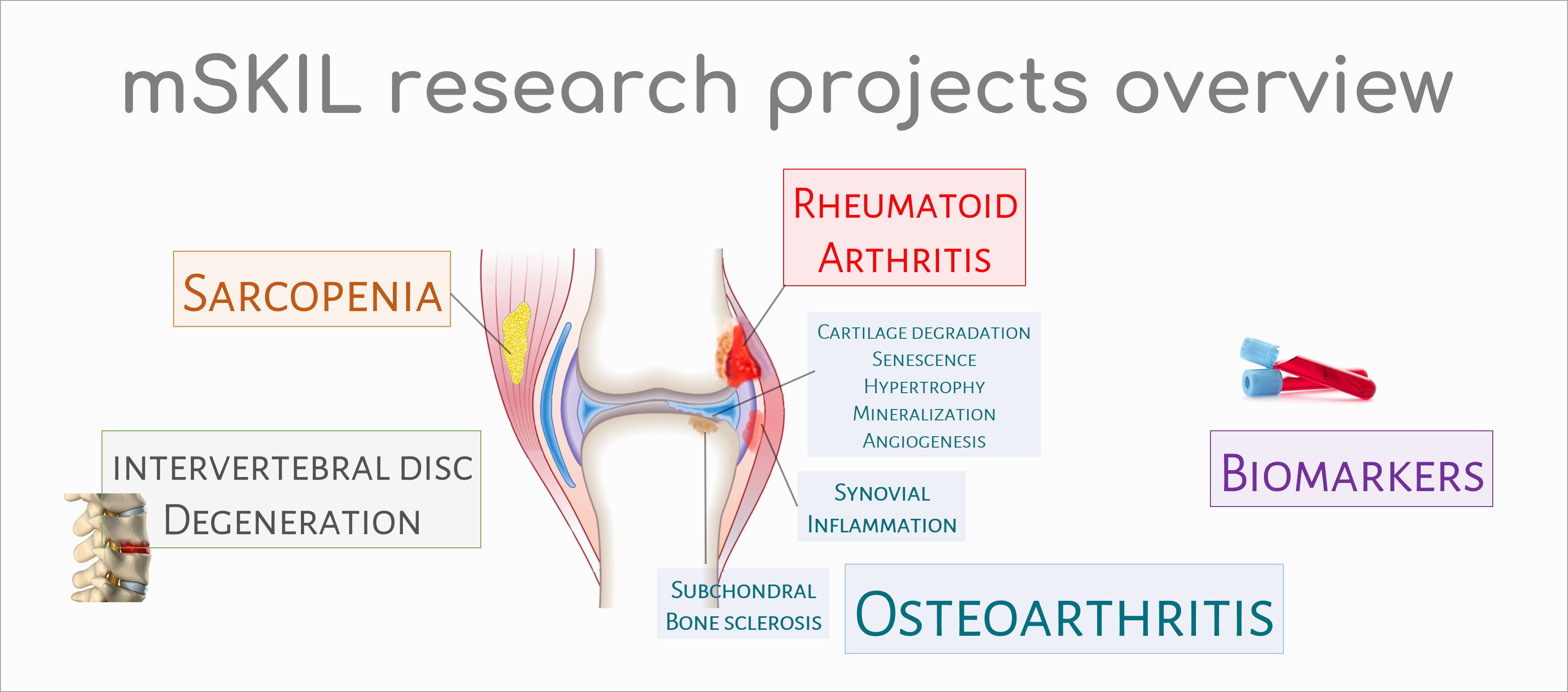
Osteoarthritis (OA) is one of the most prevalent and chronic diseases affecting the elderly. Its most prominent feature is the progressive destruction of articular cartilage, even it is now accepted that OA is a global disease involving synovial membrane, subchondral bone and periarticular soft tissues. OA may occur following traumatic injury to the joint, subsequent to an infection of the joint or simply as a result of ageing and the wear and tear associated with the stresses of daily life. Injured articular cartilage does not spontaneously repair. Besides surgical prostheses, dedicated to advanced stages of osteoarthritic (OA) lesions, no satisfactory treatment is presently available for degenerative lesions of cartilage. Our research projects also intend to develop alternative therapeutics to surgery. Over the past decade, many studies have linked cellular senescence to aging and also to OA development. The accumulation of senescent cells in tissues contributes to both aging and the promotion of age-related diseases like OA. Cellular senescence is a stress-response process that results in cells entering a stable state of growth arrest while remaining metabolically active. Senescent cells secrete a large variety of inflammatory cytokines and many factors known as the senescence-associated secretory phenotype (SASP). Indeed, senescent chondrocytes secrete high levels of IL-1α-β, IL-6, IL-8, CCL2, TNF-α, IGFBP3, MMP-1, -3 and -13. Selective removal of these senescent chondrocytes or inhibiting the senescence process are new promising approaches for OA therapy, at least in the "senescent ageing-related phenotype" subgroup of OA patients.
Normal joint cartilage contains no blood vessels. This characteristic is due to high concentrations within the cartilage of anti-angiogenic factors. In osteoarthritis, cartilage angiogenesis is involved in osteophyte development, subchondral bone remodeling, and cartilage mineralization.
OA was recently defined by the Osteoarthritis Research Society International (OARSI) as a disorder characterized by cell stress and extracellular matrix degradation initiated by micro- and macro-injury that activates maladaptive repair responses including pro-inflammatory pathways of immunity. The activation of the innate and adaptive immune systems is closely associated with the low grade systemic inflammation in OA. This process was initiated and driven in the synovial membrane, especially by synovium cells activated by damage-associated molecular patterns (DAMPs) released from cartilage during its degradation. DAMPs binds pattern-recognition receptors (PRRs) including membrane-bound receptors such as Toll-like receptors (TLRs) or RAGE receptors and cytoplasmic receptors like NOD-like receptors (NLRs). These receptors are localized either on the surface of cells (immune cells, chondrocytes and synoviocytes; TLRs - RAGE) or in the cell cytoplasm (NLRs). Receptors activation initiates then downstream signalling cascades leading to the activation of transcription factors such as nuclear factor-kB (NF-kB), a key regulator of the inflammatory response. NF-kB activation leads to the release of various factors involved in OA pathogenesis like catabolic factors (MMP-1, -3, -9 and -13), cytokines (TNF-a and IL-1β), chemokines (CCL-7, -8, IL-8) and complement factors. This also leads to macrophage and T cell infiltration in the synovial membrane and to increase of vascular permeability. In OA process, peptides from the breakdown of type II collagen have been shown to initiate the "pro-inflammatory" pathways of natural immunity. In our research unit, two peptides from type II collagen, Coll2-1 and Coll2-2, have been patented as biomarkers for the degradation of articular cartilage. We therefore looked for the effects of these peptides and in particular, the effect of Coll2-1 on joint metabolism. In vitro, we observed that this peptide has pro-inflammatory, pro-angiogenic and immunomodulatory properties directly linked to osteoarthritis and that in vivo, in the Lewis rat, this peptide is also capable of inducing polyarthritis. The normal synovium is highly vascularized to provide cartilage nutrients and oxygen. However, in osteoarthritis, the angiogenic process is increased locally. This results in a proliferation of endothelial cells and is attributed to an imbalance between pro- and anti-angiogenic factors. Inflammation and angiogenesis are two processes closely associated with the progression of osteoarthritis.
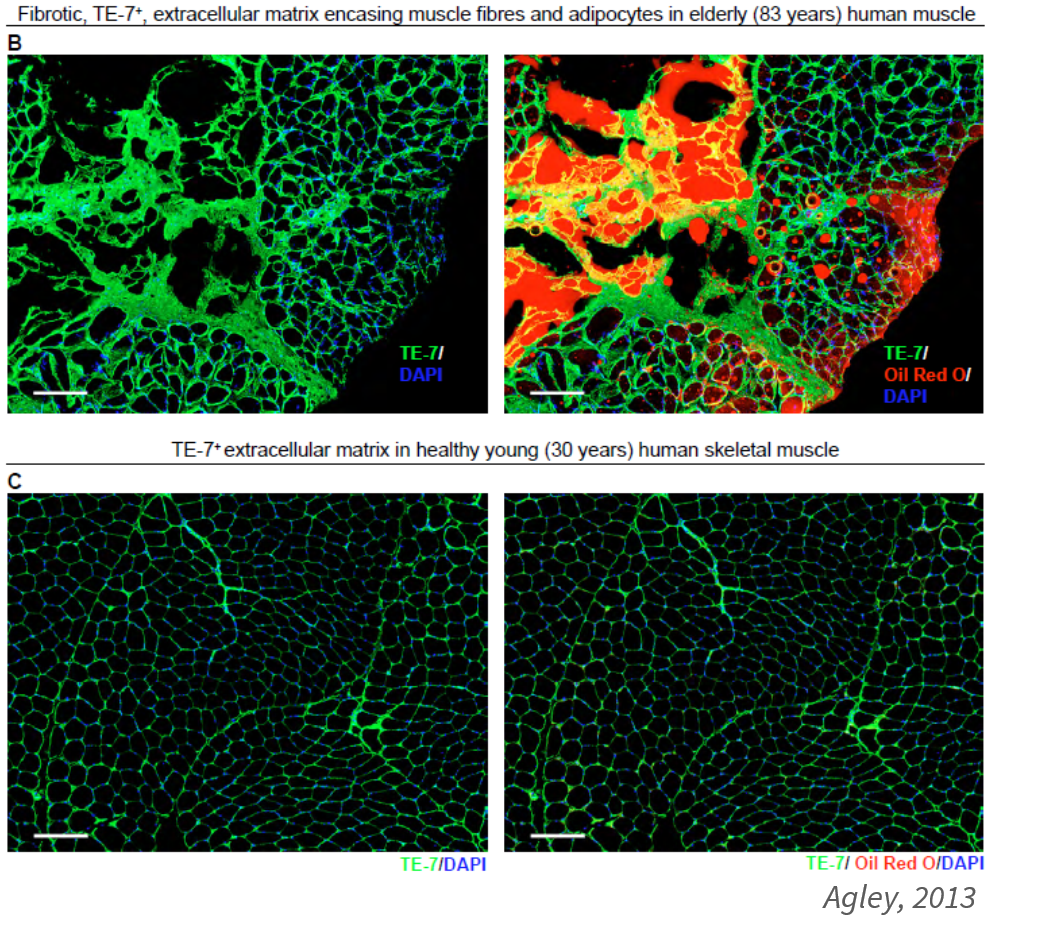
In our laboratory, we seek to characterize the inflammatory and angiogenic phenotype of fibroblast-like synoviocytes isolated from synovial tissue "normal" or "reactive" (N/R) and osteoarthritis "inflammatory" (I). Immunohistochemical analysis revealed in the I area a greater number of CD45+ (at the synovial intima and nodular inflammatory infiltrates) and VEGF+ (at the intima). The diameter of blood vessels identified by von Willebrand factor also appears greater in this area. In parallel with these results, we observed a significant increased (p<0,001) of IL-6, IL-8 and PGE2 production by synoviocytes from the I area compared to synoviocytes from N/R area. Finally, in comparison with synoviocytes of N/R area, synoviocytes of I area produced significantly more VEGF (p<0,001) but less than TSP-1 (p<0,001). Sarcopenia is a complex, multifactorial, age-related skeletal muscle disorder. Currently, it is defined by the measure of 3 parameters: muscle strength, muscle quantity/quality and physical performance. The global prevalence of sarcopenia among people aged between 60 and 70 is between 5 and 13%, and increases between 11 and 50% for people over 80 years of age. Sarcopenia muscle contains more adipocytes and fibrous extracellular matrix than healthy muscle.
In the laboratory, we developed a model of skeletal muscle cell culture. The aim is to analyze the secretome of these myotubes by mass spectrometry in order to identify possible biomarkers of sarcopenia.
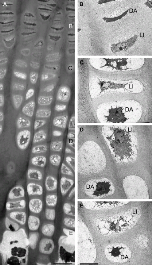
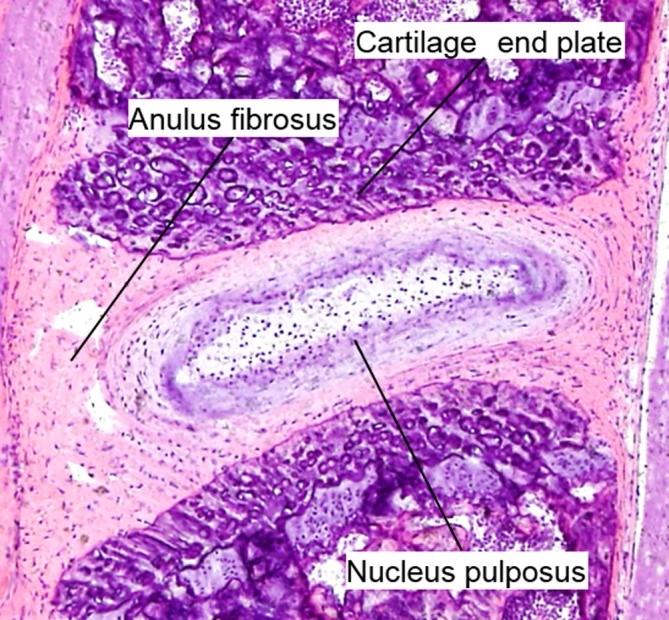
Low back pain is a common condition affecting approximately 637 million individuals worldwide (James S et al., Lancet 2018). Intervertebral disc degeneration (IDD) is one of the main causes of low back pain associated with lower health-related quality of life and high medical expenses, resulting in increased suffering and high socioeconomic costs (Oichi T et al., JOR spine 2020). IDD has many risk factors among which genetic factors, aging, mechanical stress and injury play an important role. The imbalance between catabolic and anabolic activities and changes in extracellular matrix (ECM) characterized by the changes in the expression/structure of collagens/proteoglycans (increase of type I collagen and decrease in both proteoglycan and type II collagen) are the pathophysiological mechanisms. Histological changes result in fissure, cell cluster formation, increased degree of vascularization and innervation, mineralization or microfracture and slerosis of subchondral bone (Oichi T et al., 2020). Several animal models have been developped to elucidate the pathomechanism of IDD. In this context, a lumbar disc degeneration model is currently under development in our research unit by needle punctures to the discs in Sprague-Dawley rats.
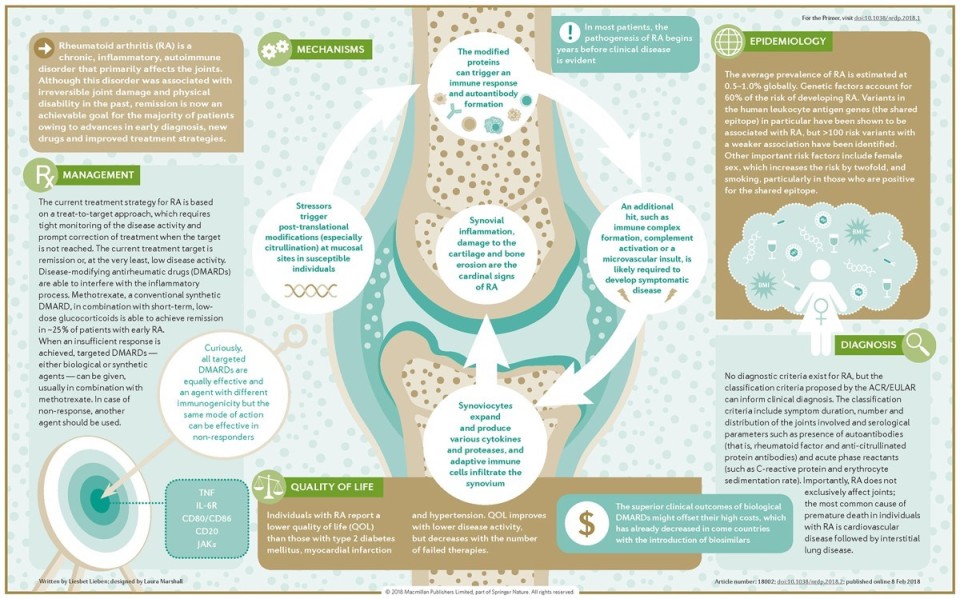
Hematoxylin and eosin staining of mouse lumbar intervertebral disc at 8 weeks of age (Oichi et al, 2020). Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune dissease characterized by autoantibodies to immunoglobulin G (IgG- rheumatoid factor-RF) and citrullinated proteins (anti-citrullinated protein antibodies-ACPAs). This pathology is associated with several risk factors including genetics, female sex and environmental factors. Briefly, the adaptative immunity recognize neo-epitopes following post-translational modifications such as citrullination in the mucosa (mouth, lung and gut). These altered peptides are presented by antigen-presenting cells (APCs), then activate the adaptative immune response in lymphoid tissues and finally induce autoantibody formation. In return, fibroblast-like synoviocytes, APCs and macrophages activate and produce a lot of number inflammatory factors. These latter induce the synovial inflammation and the production of cytokines, chemokines, leukotriens, prostaglandins, MMPs. Paracrine and autocrine actions of these cytokines and persistent adaptative immune responses can perpetuate the disease and lead to cartilage and bone destruction.
Nature Reviews Disease Primers volume 4, Article number: 18002 (2018) The National Institutes of Health (NIH) Biomarkers Definitions Working Group defined a biomarker as "a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention".
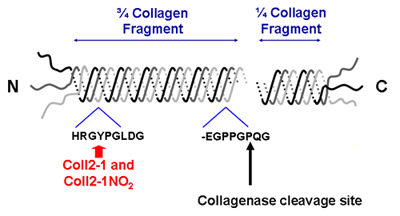
Clinical OA is now considered to be preceded by a "silent" pre-radiographic phase during which extensive metabolic changes occur in joint tissues, without any pain. One challenge is the detection of these early metabolic changes that are early indicators of abnormal joint changes before the occurrence of structural changes. We started to focus ou research on OA biomarkers in early 2000's. We used a new approach for quantifying type II collagen network degradation. This approach is based on the detection in urine, serum and synovial fluid of a peptide released from the triple helix of type II collagen in its native or nitrated form. Peptide nitration results from the reaction of aromatic amino acids with peroxynitrite (ONOO-), a strong oxidant formed by the reaction of nitric oxide (●NO) and superoxide anion (O2-). In OA, ●NO and O2- are produced by a large number of cells including macrophages and chondrocytes. Tyrosine, tryptophan and phenylalanine are particularly sensitive to nitration. Type II collagen contains two tyrosines, one located in the triple helix and the other in the telopeptide at the C-terminal end.
According the criteria of BIPED*classification, Coll2-1 and Coll2-1NO2 are a useful biomarker for the classification of individuals as diseased or no diseased, the assessment of severity or extent of osteoarthritis and the prediction of future onset or progression of osteoarthritis. See our recent review on these biomarkers here : Coll2-1 and Coll2-1NO2 as exemplars of collagen extracellular matrix turnover - biomarkers to facilitate the treatment of osteoarthritis? Ali Mobasheri, Cecile Lambert & Yves Henrotin Expert Review of Molecular Diagnostics 2019 These biomarkers are now available at Artialis SA. *The BIPED (B = burden of disease; I = investigative; P = prognostic; E = efficacy of intervention; D = diagnostic) classification was proposed by the Osteoarthritis Biomarkers Network (a consortium of five US National Institutes of Health designated sites) Bauer DC et al. Osteoarthritis cart 2006 Differential proteomics provide a powerful tool to identify new biomarkers of osteoarthritis. This approach is not restricted to consider proteins derived from the degradation of cartilage.
It allows the identification of specific proteins whose concentration is modified significantly in different biological from patients with OA.
The pattern of expression of these proteins in a particular biological compartment can therefore serve as a biological marker of the disease. Using this method on urine and on cell-culture supernatants, we have discovered
fibulin-3 and osteomodulin peptides as new potential OA biomarkers.
Thanks to an Excellence Of Science (EOS) grant obtained from the FNRS/FWO, we continue our research on these fibulin-3, type II collagen nitrated and osteomodulin peptides as OA biomarkers.
The current diagnosis is based on a combination of physical criteria (physical performance and muscle strength tests) associated with medical imaging (determination of muscle mass). This clinical diagnosis is expensive, imprecise and tough for the patient. Therefore, the use of specific biomarkers could be a simple, fast and inexpensive method for the diagnosis and monitoring of sarcopenia.
|
||||||||||||||||||||||||