Using primary cells, functional cellular assays are available for the screening and mechanistic investigation of the biological activities of treatments on synovial cells, articular chondrocytes, osteoblasts and myoblasts. We can perform these studies using our different innovative in vitro models, as close as possible to the real in vivo context. We can propose a large panel of cell culture models : Embedded in a porous alginate matrix, chondrocytes maintain a spherical shape, without cell to cell contact in a matrix close to cartilage. In the absence of serum, primary articular chondrocytes keep their phenotype in cell culture for at least five weeks. The matrix provides a compartmentalization identical to that found in the hyaline cartilage in vivo, with a cell matrix and a further-removed matrix like in normal cartilage. This model is ideal for studying matrix, enzymes and cytokines production, and for assessing long-term treatments.
Sanchez C, Hemmer K, Krömmelbein N, Seilheimer B, Dubuc JE, Antoine C, Henrotin Y: Reduction of Matrix Metallopeptidase 13 and Promotion of Chondrogenesis by Zeel T in Primary Human Osteoarthritic Chondrocytes. Frontiers in Pharmacology 2021, 1201-12. Sanchez, C., Lambert, C., Dubuc, J.-E., Bertrand, J., Pap, T., & Henrotin, Y: Syndecan-4 is Increased in Osteoarthritic Knee, but not Hip or Shoulder, Articular Hypertrophic Chondrocytes. Cartilage 2021, 13:862-871. Oprenyeszk F, Sanchez C, Dubuc JE, Maquet V, Henrist C, Compere P, Henrotin Y: Chitosan enriched three-dimensional matrix reduces inflammatory and catabolic mediators production by human chondrocytes. PLoS One 2015, 10(5):e0128362. Pesesse L, Sanchez C, Delcour JP, Bellahcene A, Baudouin C, Msika P, Henrotin Y: Consequences of chondrocyte hypertrophy on osteoarthritic cartilage: potential effect on angiogenesis. Osteoarthritis Cartilage 2013, 21(12):1913-1923. Sanchez C, Deberg MA, Burton S, Devel P, Reginster JY, Henrotin YE: Differential regulation of chondrocyte metabolism by oncostatin M and interleukin-6. Osteoarthritis Cartilage 2004, 12(10):801-810. Sanchez C, Mathy-Hartert M, Deberg MA, Ficheux H, Reginster JY, Henrotin YE: Effects of rhein on human articular chondrocytes in alginate beads. Biochem Pharmacol 2003, 65(3):377-388. Henrotin YE, Sanchez C, Deberg MA, Piccardi N, Guillou GB, Msika P, Reginster JY: Avocado/soybean unsaponifiables increase aggrecan synthesis and reduce catabolic and proinflammatory mediator production by human osteoarthritic chondrocytes. J Rheumatol 2003, 30(8):1825-1834. Sanchez C, Mateus MM, Defresne MP, Crielaard JM, Reginster JY, Henrotin YE: Metabolism of human articular chondrocytes cultured in alginate beads. Longterm effects of interleukin 1beta and nonsteroidal antiinflammatory drugs. J Rheumatol 2002, 29(4):772-782. Human or bovine articular chondrocytes can also be cultured in monolayer. This model is ideal for the screening of a large panel of drug candidates based on a large panel of well-characterized items like cellular proliferation, apoptosis and genes involved in cartilage remodeling or inflammation. Comblain, F., Dubuc, J.-E., Lambert, C., Sanchez, C., Lesponne, I., Serisier, S., & Henrotin, Y. Identification of Targets of a New Nutritional Mixture for Osteoarthritis Management Composed by Curcuminoids Extract, Hydrolyzed Collagen and Green Tea Extract. PLoS One 2016. Comblain F, Sanchez C, Lesponne I, Balligand M, Serisier S, Henrotin Y: Curcuminoids extract, hydrolyzed collagen and green tea extract synergically inhibit inflammatory and catabolic mediator's synthesis by normal bovine and osteoarthritic human chondrocytes in monolayer. PLoS One 2015, 10(3):e0121654. Mathy-Hartert M, Jacquemond-Collet I, Priem F, Sanchez C, Lambert C, Henrotin Y: Curcumin inhibits pro-inflammatory mediators and metalloproteinase-3 production by chondrocytes. Inflamm Res 2009, 58(12):899-908. Explant culture is ideal to investigate the matrix degradation. Embedded in a porous alginate matrix and cultured in the presence of fetal bovine serum, articular OA chondrocytes undergo a hypertrophic differentiation 3 to 4 weeks after culture initiation. As in hypertrophic differentiation observed during OA, chondrocytes start to express MMP-13, type X collagen and highly increased their alkaline phosphatase activity. Sanchez, C., Lambert, C., Dubuc, J.-E., Bertrand, J., Pap, T., & Henrotin, Y. (in press). Syndecan-4 is Increased in Osteoarthritic Knee, but not Hip or Shoulder, Articular Hypertrophic Chondrocytes. Cartilage. Pesesse L, Sanchez C, Delcour JP, Bellahcene A, Baudouin C, Msika P, Henrotin Y: Consequences of chondrocyte hypertrophy on osteoarthritic cartilage: potential effect on angiogenesis. Osteoarthritis Cartilage 2013, 21(12):1913-1923. Chondrocytes senescence is associated to OA and lead to cartilage degradation and release of inflammatory mediators. To prevent senescence or to induce senescent cells lysis are therapeutic targets for anti-OA compounds. Chondrocyte senescence can be induced using a sub-lethal oxidative stress, which mimic the oxidative stress occurring in OA cartilage. Senescence is investigated using a panel of relevant markers including HMGB2, IL-6. In this model, osteoblasts coming from either sclerotic or non-sclerotic zones of human OA subchondral bone are cultured separately and their endotype compared using a large serie of markers. Therefore, responses of sclerotic and non-sclerotic osteoblasts to biochemical or mechanical stimuli can be compared. Furthermore this model can be used in an original co-culture model, in which subchondral osteoblasts in monolayer are cultured together with osteoarthritis chondrocytes in alginate beads. It's a useful tool to evaluate the potential of new drugs to normalize bone remodeling in OA subchondral bone. Sanchez, C., Mazzucchelli, G., Lambert, C., Comblain, F., De Pauw, E., & Henrotin, Y. (2018). Comparison of secretome from osteoblasts derived from sclerotic versus non-sclerotic subchondral bone in OA: A pilot study. PLoS ONE, 13(3), 0194591. Sanchez, C., Horcajada, M.-N., Membrez Scalfo, F., Ameye, L., Offord, E., & Henrotin, Y. (2015). Carnosol Inhibits Pro-Inflammatory and Catabolic Mediators of Cartilage Breakdown in Human Osteoarthritic Chondrocytes and Mediates Cross-Talk between Subchondral Bone Osteoblasts and Chondrocytes. PLoS ONE, 10(8), 0136118. Sanchez C, Deberg MA, Bellahcene A, Castronovo V, Msika P, Delcour JP, Crielaard JM, Henrotin YE: Phenotypic characterization of osteoblasts from the sclerotic zones of osteoarthritic subchondral bone. Arthritis Rheum 2008, 58(2):442-455. Henrotin YE, Deberg MA, Crielaard JM, Piccardi N, Msika P, Sanchez C: Avocado/soybean unsaponifiables prevent the inhibitory effect of osteoarthritic subchondral osteoblasts on aggrecan and type II collagen synthesis by chondrocytes. J Rheumatol 2006, 33(8):1668-1678. Sanchez C, Deberg MA, Piccardi N, Msika P, Reginster JY, Henrotin Y: Osteoblasts from the sclerotic subchondral bone downregulate aggrecan but upregulate metalloproteinases expression by chondrocytes. This effect is mimicked by Interleukin-6, -1β and Oncostatin M pre-treated non sclerotic osteoblasts. Osteoarthritis Cartilage 2005, 13(11):979-987. Sanchez C, Deberg MA, Piccardi N, Msika P, Reginster JY, Henrotin Y: Subchondral bone osteoblasts induce phenotypic changes in human osteoarthritic chondrocytes. Osteoarthritis Cartilage 2005, 13(11):988-997. In this original model, synoviocytes coming from the inflammatory and non-inflammatory zone of human OA synovial membrane are cultured separately and their metabolism compared using a large panel of biomarkers associated to angiogenesis, fibrosis or inflammation. This model consists of selecting samples from normal/reactive or inflamed areas from the same OA patient using the Ayral synovitis score, and thereafter to culture cells separately in monolayer in a well-defined culture medium.
Lambert C, Dubuc JE, Montell E, Vergés J, Munaut C, Noël A, Henrotin Y. Gene expression pattern of cells from inflamed and normal areas of osteoarthritis synovial membrane. Arthritis Rheumatol. 2014; 66(4):960-8. Lambert C, Mathy-Hartert M, Dubuc JE, Montell E, Vergés J, Munaut C, Noël A, Henrotin Y. Characterization of synovial angiogenesis in osteoarthritis patients and its modulation by chondroitin sulfate. Arthritis Res Ther. 2012; 14(2):R58.
This model of skeletal muscle cell culture was developed for a better understanding of the pathophysiological mechanisms of sarcopenia. Briefly, muscle biopsies undergo enzymatic digestion to release cells from the extracellular matrix. Fibroblasts and myoblasts are then separated with immunomagnetic beads. Myoblasts are then differentiated into myotubes thanks to well-defined and original culture conditions. Through this model we can study the proliferation and the differentiation of skeletal muscle cells and the impact of a potential therapeutic agent on these two
EORS 2019, Maastricht, The Netherlands, October 2019 " Development of a muscle cell culture model from human muscle biopsies" Florin, A., Lambert, C., Sanchez, C., Zappia, J., Durieux, N., Tieppo, A. M., Mobasheri, A., & Henrotin, Y. (2020, January 02). The secretome of skeletal muscle cells: A systematic review. Osteoarthritis and Cartilage Open. During movement, chondrocytes, synoviocytes, osteoblasts and myoblasts are subjected to tensile stress resulting from the sliding of articular surfaces. The Flexercell FX4000 is a computer-regulated bioreactor that uses vacuum pressure to apply cyclic or static strain to cells cultured on flexible-bottomed culture plates. The force exerted on the cells and its frequency can be modulated. This model allows the study of traction or compression on the cellular metabolism. Discover the device in our technological platform For example, our innovative three-dimensional in vitro model allows the study of cyclic compression on osteoblasts, embedded in a newly synthesized extracellular matrix. In this model, cell/matrix interactions are conserved and fluid flow through a three-dimensional extracellular matrix is allowed.
Sanchez C, Gabay O, Salvat C, Henrotin YE, Berenbaum F: Mechanical loading highly increases IL-6 production and decreases OPG expression by osteoblasts. Osteoarthritis Cartilage 2008. Sanchez C, Gabay O, Henrotin YE, Berenbaum F: Osteoblast: A cell under compression. Biomed Mater Eng 2008, 18(4-5):221-224. We propose a large panel of animal models dedicated to the study of bone remodeling, inflammation, pain, cartilage degradation and muscle atrophy. In addition, we also possess a collection of validated and qualified original biomarkers. Moreover, different methods are available to study animals behavior and tissues modification associated with different disease conditions.
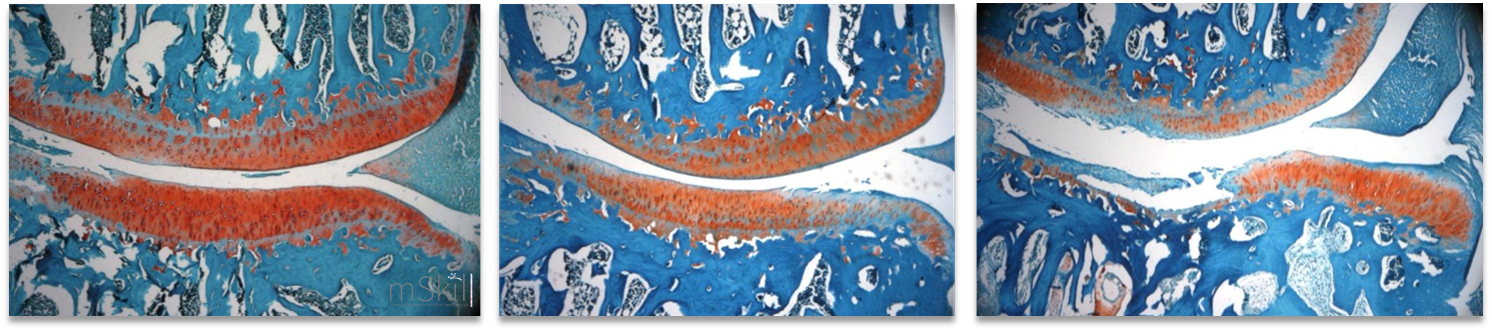
This model has a histopathological similarity to human disease. The joint pathology in both guinea pig and human is age-related and linked to a diversity of risk factors such as body weight, mechanical load and high bone turnover. This model is characterized by an early collagen fibril disruption occurring in articular cartilage, bone cysts, subchondral bone thickening and osteophytes are preceding histological proteoglycan loss and fibrillation.
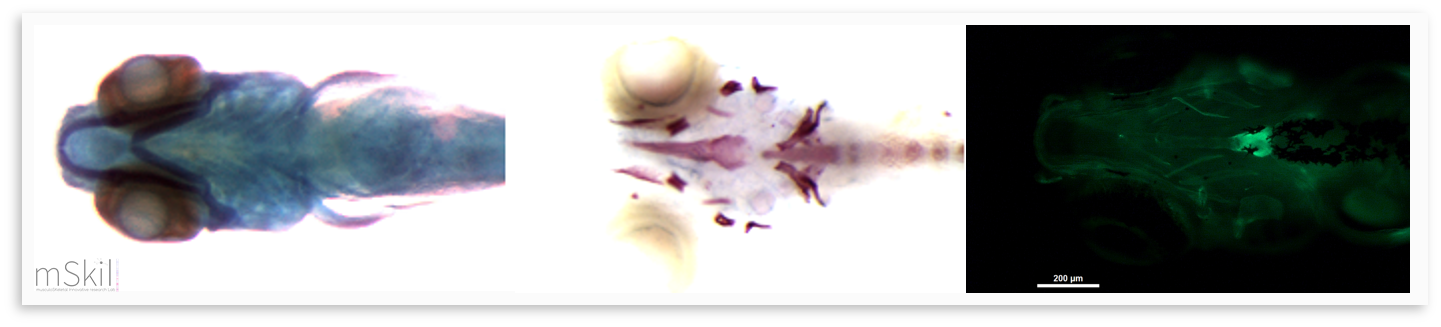
Horcajada MN, Sanchez C, Membrez Scalfo F, Drion P, Comblain F, Taralla S, Donneau AF, Offord EA, Henrotin Y. Oleuropein or rutin consumption decreases the spontaneous development of osteoarthritis in the Hartley guinea pig. Osteoarthritis Cartilage. 2015 Jan;23(1):94-102. The surgical destabilization of the medial meniscus (DMM) model mimics clinical meniscal injury, and the surgical anterior cruciate ligament section can be made in mouse, rat and rabbit. These models are useful for studying the onset and progression of posttraumatic and mechanically induced- osteoarthritis. We also offer biological markers, helping in the detection of metabolic dysfunctions of cartilages before histologic lesions. Native Type II collagen injected subcutaneously (CIA) or SCW extract PeptidoGlycan-PolySaccharide (PG-PS) injected into the joint are considered as reference models for the study of arthritis. These models offer biological markers, helping in the detection of metabolic dysfunctions of cartilages before histologic lesions. Lambert, C., Borderie, D., Dubuc, J.-E., Rannou, F., & Henrotin, Y. (2019). Type II collagen peptide Coll2-1 is an actor of synovitis. Osteoarthritis and Cartilage, 27(11), 1680-1691. The zebrafish is a model organism which has been used since the 1960s but whose use really took off in the 1980s. The reasons for its success are: its ease of maintenance, rapid development, its abundant offspring, its genome is sequenced and can easily be modified by genetic engineering, the larvae are transparent which is ideal for in vivo observations. Also, the zebrafish demonstrates 70% of genes that are orthologous to those of humans and more than 80% of human genes associated with pathologies have at least one ortholog in the zebrafish (Howe et al., 2013). The zebrafish remains relevant for joint pathologies studies since it presents the various joints including the synovial joints (Askary et al., 2016).
Using this model, we can generate and study mutants for genes that we believe to be involved in osteoarthritis and we can characterize the in vivo effects on skeletal tissue. Several genes implicated in osteoarthritis are or have been studied within the zebrafish and have demonstrated its usefulness as a model in the study of this pathology (Lawrence et al., 2018; Mitchell et al., 2013). Inflammation and its role in osteoarthritis have also been clarified using this model (Jurynec et al., 2018). Askary, A., Smeeton, J., Paul, S., Schindler, S., Braasch, I., Ellis, N. A.,... Gage Crump, J. (2016). Ancient origin of lubricated joints in bony vertebrates. https://doi.org/10.7554/eLife.16415.001 Howe, K., Clark, M. D., Torroja, C. F., Torrance, J., Berthelot, C., Muffato, M., ... Stemple, D. L. (2013). The zebrafish reference genome sequence and its relationship to the human genome. Nature. https://doi.org/10.1038/nature12111 Jurynec, M. J., Sawitzke, A. D., Beals, T. C., Redd, M. J., Stevens, J., Otterud, B., ... Grunwald, D. J. (n.d.). A hyperactivating proinflammatory RIPK2 allele associated with early-onset osteoarthritis. https://doi.org/10.1093/hmg/ddy132 Lawrence, E. A., Kague, E., Aggleton, J. A., Harniman, R. L., & Roddy, K. A., Hammond, C. L. (2018). The mechanical impact of col11a2 loss on joints; col11a2 mutant zebrafish show changes to joint development and function, which leads to early-onset osteoarthritis. https://doi.org/10.1098/rstb.2017.0335 Mitchell, R. E., Huitema, L. F. A., Skinner, R. E. H., Brunt, L. H., Severn, C., Schulte-Merker, S., & Hammond Yz, C. L. (2013). New tools for studying osteoarthritis genetics in zebrafish. Osteoarthritis and Cartilage, 21, 269-278. https://doi.org/10.1016/j.joca.2012.11.004
Intervertebral disc degeneration (IDD) related lower back pain affects between 60% and 80% of the population, making it one of the important public health issues. The major pathological manifestations of IDD are the progressive declining proteoglycan content, disappearance of nucleus pulposus and collapse of annulus fibrosus. The height of the intervertebral discs is also reduced and osteophytes appear at the anterior and lateral edges of the disc. The challenge today is to develop a therapeutic method capable of restoring the functions of the disc. In this context, a lumbar disc degeneration model is currently under development in our research unit by needle punctures to the discs in Sprague-Dawley rats.
|
||||||||||||||











 Sanchez C, Zappia J, Lambert C, Foguenne J, Dierckxsens Y, Dubuc JE, Delcour JP, Gothot A & Henrotin, Y.: Curcuma longa and Boswellia serrata Extracts Modulate Different and Complementary Pathways on Human Chondrocytes In Vitro: Deciphering of a Transcriptomic Study. Frontiers in Pharmacology 2022, 13.
Sanchez C, Zappia J, Lambert C, Foguenne J, Dierckxsens Y, Dubuc JE, Delcour JP, Gothot A & Henrotin, Y.: Curcuma longa and Boswellia serrata Extracts Modulate Different and Complementary Pathways on Human Chondrocytes In Vitro: Deciphering of a Transcriptomic Study. Frontiers in Pharmacology 2022, 13. Lambert, C., Borderie, D., Dubuc, J.-E., Rannou, F., & Henrotin, Y. (2019). Type II collagen peptide Coll2-1 is an actor of synovitis. Osteoarthritis and Cartilage, 27(11), 1680-1691.
Lambert, C., Borderie, D., Dubuc, J.-E., Rannou, F., & Henrotin, Y. (2019). Type II collagen peptide Coll2-1 is an actor of synovitis. Osteoarthritis and Cartilage, 27(11), 1680-1691. crucial steps of myogenesis (doubling time of myoblasts, myogenic index, myotubes area). A large number of muscle specific markers can also be investigated (Desmin, Myf5, Myf6, MYH1, MYH2, MYH3, MyoD1, Myogenin, Pax7, Ki-67, MYH7, MYH6, MYH, Ptx3,...), with gene expression profiling, protein secretion, flow cytometry and immunofluorescence analysis. Since the skeletal muscle is an endocrine organ, we are also able to study the secretome of fibroblasts, myoblasts and / or myotubes.
crucial steps of myogenesis (doubling time of myoblasts, myogenic index, myotubes area). A large number of muscle specific markers can also be investigated (Desmin, Myf5, Myf6, MYH1, MYH2, MYH3, MyoD1, Myogenin, Pax7, Ki-67, MYH7, MYH6, MYH, Ptx3,...), with gene expression profiling, protein secretion, flow cytometry and immunofluorescence analysis. Since the skeletal muscle is an endocrine organ, we are also able to study the secretome of fibroblasts, myoblasts and / or myotubes.
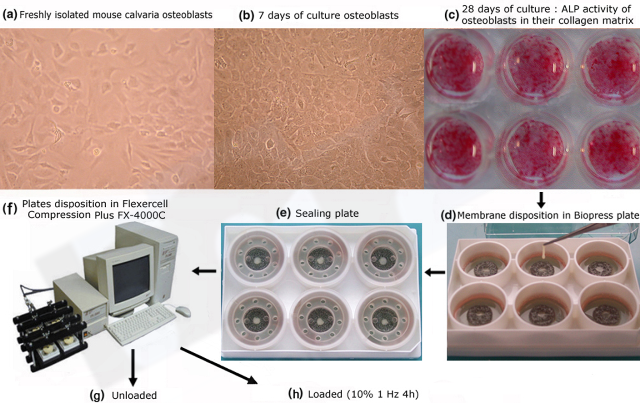 Sanchez C, Pesesse L, Gabay O, Delcour JP, Msika P, Baudouin C, Henrotin YE: Regulation of subchondral bone osteoblast metabolism by cyclic compression. Arthritis Rheum 2012, 64(4):1193-1203.
Sanchez C, Pesesse L, Gabay O, Delcour JP, Msika P, Baudouin C, Henrotin YE: Regulation of subchondral bone osteoblast metabolism by cyclic compression. Arthritis Rheum 2012, 64(4):1193-1203.